Abstract
Background: Long-term prospective studies (5-yr follow-up) report a 16-17% risk of cataract development in a general population aged >40 yrs (Klein BE e t al. Ophthalmology 2002; McCarty CA et al. Am J Ophthalmol 2003). EPAG is an oral thrombopoietin receptor agonist approved for the treatment of pts with cITP aged ≥1 yr, who have had an insufficient response to prior therapy (eg corticosteroids, immunoglobulins). Clinical trials of EPAG of up to 6-months' duration reported cataract development/worsening in 7% of both EPAG-treated and placebo pts (PROMACTA prescribing information, Novartis, July 2017). EXTEND was a global, open-label, long-term extension study of pts with cITP who received EPAG or placebo in prior EPAG studies, and evaluated long-term safety and tolerability. We describe the occurrence and management of cataracts in pts with ITP during EPAG treatment in EXTEND.
Methods: Adult cITP pts started EPAG at 50 mg/day, titrating to 25-75 mg/day or less often as required, based on a platelet count target range ≥50-≤200×109/L. Maintenance dosing continued after minimization of concomitant ITP medications (if any) eg, steroids and optimization of EPAG dosing. Pts could remain on EPAG for 2 yrs in countries where EPAG was commercially available or >2 yrs until EPAG became commercially available. EXTEND described the long-term safety and tolerability of EPAG by monitoring relevant parameters, eg, ocular exams. Ocular exams were performed by ophthalmologists/optometrists at enrollment and every 12 months during EXTEND. The protocol defined ocular events of 'clinical concern' as serious adverse events (SAEs).
Results: 302 pts enrolled and received ≥1 EPAG dose: 67% female; 17% ≥65 yrs old (median, 50). Median exposure duration was 2.4 yrs (range, 2 days to 8.8 yrs) and mean daily dose was 50.2 mg/day (SD, 21.6). 245/302 pts (81%) received corticosteroid treatment before entering EXTEND; 89/302 (29%) patients initiated EXTEND while on concomitant corticosteroids. Overall, 259/302 (86%) pts achieved platelet counts ≥50×109/L at least once and 126/248 (51%) pts maintained continuous counts ≥50×109/L for ≥31 wks.
At enrollment, 192/291 (66%) pts assessed had ≥1 cataract risk factor: long-term corticosteroid use (n=142; 49%), cigarette use (n=51; 18%), and/or diabetes mellitus (n=34; 12%). At enrollment, 33 (11%) pts had cataracts; of these pts, 12/33 (36%) experienced additional cataract AEs during EXTEND: worsening, n=11; non-worsening requiring surgery, n=1; resolved, n=8 (surgery, n=5; not listed, n=3); all remained on EPAG.
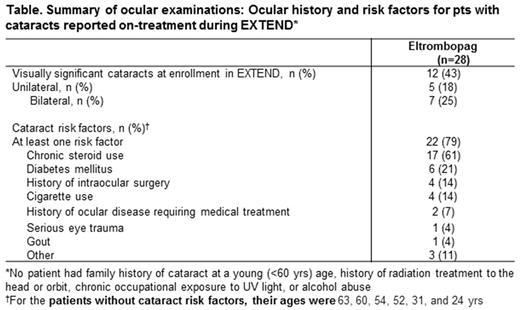
During EXTEND, 32 AEs of cataracts (worsening/ de novo) were reported in 28 (9%) pts and considered SAEs in 16 (5%). In total, 50% (16/32) of cataracts recorded during EXTEND were resolved (surgery, n=9; not listed, n=7) and 78% (25/32) required no change in EPAG dose; 1 (3%) incidence of cataract required dose interruption (not resolved) and 4 pts withdrew from the study due to cataracts (resolved, n=2/4; reason not listed). Most cataracts were mild-moderate severity (Grade 1, n=16 [50%]; Grade 2, n=9 [28%]; Grade 3, n=5 [16%]). The median time to cataract onset from first dose in EXTEND was 316 days (range, 43 days-5.6 yrs), occurring at a median age of 63 yrs (range, 25-79). 15 cataracts were reported as related to EPAG; 9/15 were resolved (surgery, n=5; not listed, n=4), 7/15 without an adjustment in dose. Of the 28 pts with cataracts during EXTEND, 18 were aged ≥60, and 10 were aged <60 yrs. At least one risk factor for cataract was recorded in 22/28 (79%) pts with cataracts, most commonly (>10%) chronic steroid use (n=17, 61%), diabetes mellitus (n=6, 21%), history of intraocular surgery (n=4, 14%), and cigarette use (n=4, 14%; Table).
Summary/conclusions: The rate of cataract development during EPAG treatment was low, and no higher (9%) than estimates in a general population aged >40 yrs (~17% over 5-yr follow-up). Together, these data do not suggest a clear link between EPAG treatment and a risk for cataract development/progression in pts with cITP. This analysis is complicated by pts' multiple confounding risk factors (eg, steroid use, diabetes, age >60 yrs and smoking). Despite the lack of clear evidence for an adverse effect of EPAG on cataract development, screening and monitoring of pts for cataracts is advised prior to initiating and during EPAG treatment, as in this study, particularly for pts with one or more risk factors.
Wong: Pfizer: Research Funding; Merck Sharp & Dohme: Research Funding; Johnson & Johnson: Research Funding; GlaxoSmithKline: Research Funding; Bristol-Myers Squibb: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Biogen-Idec: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Consultancy, Research Funding; Roche: Research Funding. Khelif: GSK: Research Funding. Saleh: GSK: Consultancy, Research Funding, Speakers Bureau. Arikan: Novartis: Employment. Quebe-Fehling: Novartis: Employment. Bussel: Boehringer Ingleheim: Research Funding; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; UpToDate: Honoraria, Patents & Royalties; Momenta: Membership on an entity's Board of Directors or advisory committees; Rigel: Membership on an entity's Board of Directors or advisory committees, Research Funding; Protalex: Membership on an entity's Board of Directors or advisory committees, Research Funding; Prophylix: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Physicians Education Resource: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal